Pfizer, a leading pharmaceutical company, has announced positive results from a midstage trial of their experimental drug for cancer cachexia, a condition that causes cancer patients to lose their appetite and weight. The drug, called ponsegromab, showed improvements in body weight, muscle mass, quality of life, and physical function in patients with cancer cachexia. This breakthrough could potentially lead to the first approved treatment for cancer cachexia in the U.S.
Cancer cachexia affects approximately 9 million people worldwide, and 80% of cancer patients with this condition are expected to die within a year of diagnosis. Patients with cancer cachexia experience significant fat and muscle loss, leaving them weak, fatigued, and unable to perform daily activities. These symptoms can also make cancer treatments less effective and contribute to lower survival rates.
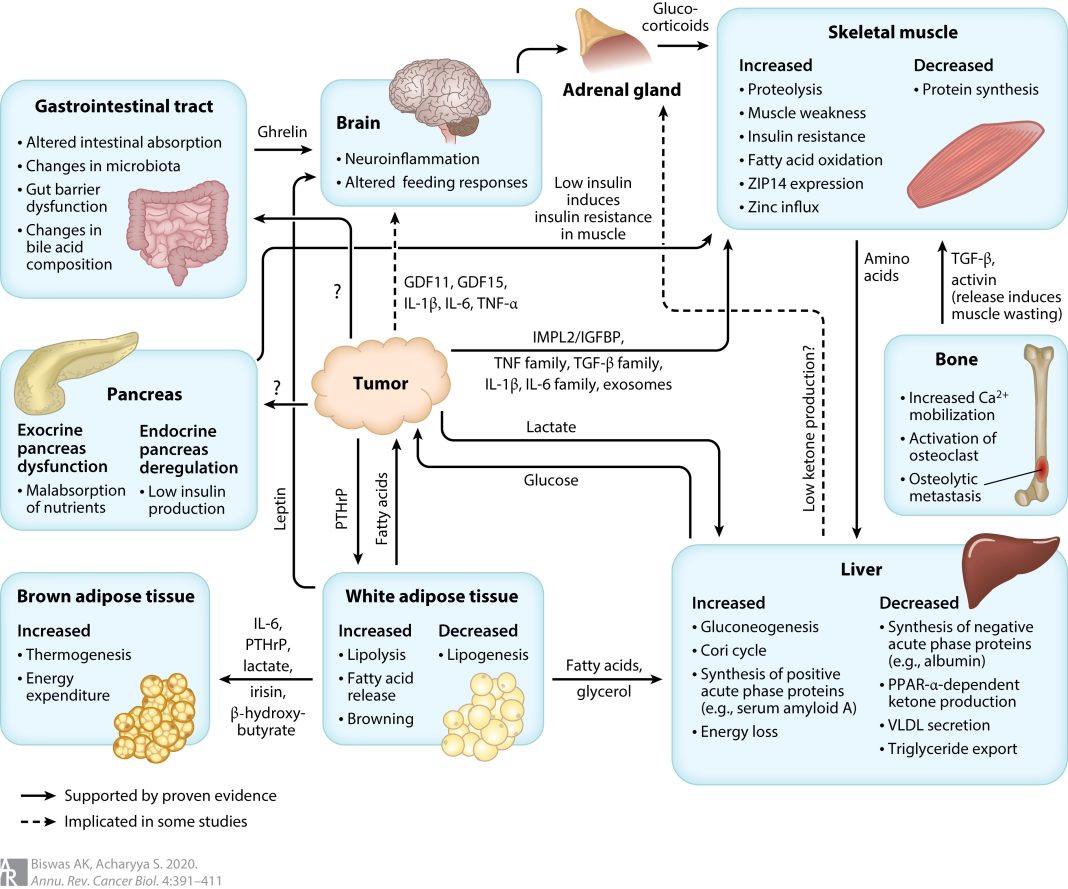
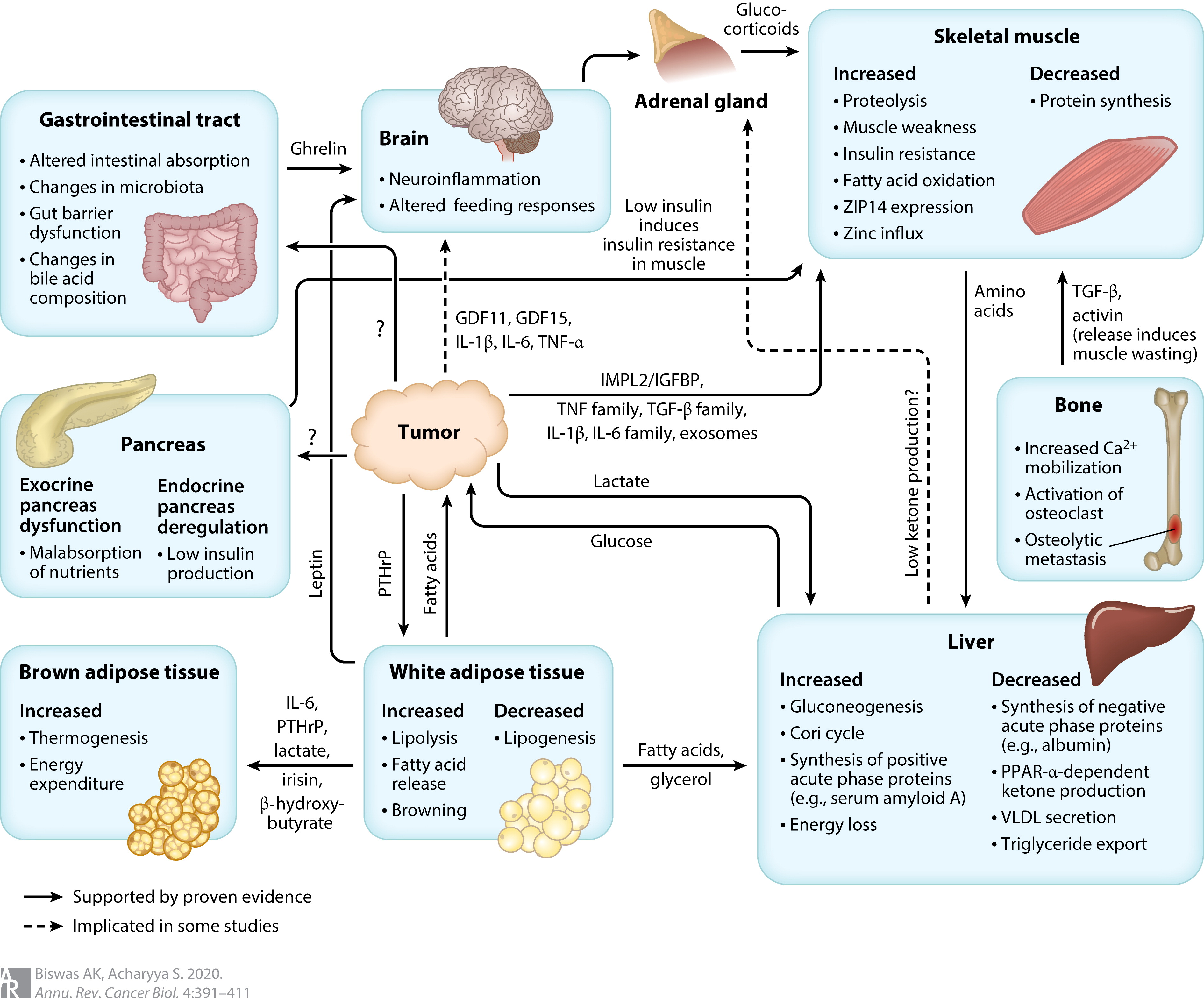
Pfizer’s head of discovery and early development, Charlotte Allerton, explained that ponsegromab could address the unmet need in cachexia by improving patients’ wellness, ability to care for themselves, and tolerance for more treatment. The drug works by reducing the levels of a protein called growth differentiation factor 15 (GDF-15), which affects appetite and is a key driver of cachexia.
The phase two trial involved 187 people with non-small cell lung cancer, pancreatic cancer, or colorectal cancer, all of whom had high levels of GDF-15. After 12 weeks, patients who took the highest dose of ponsegromab saw a 5.6% increase in weight compared to those who received a placebo. Patients who took lower doses also experienced weight gain, with a 3.5% increase for the 200-milligram dose and a 2% increase for the 100-milligram dose.
According to Allerton, a weight gain of greater than 5% is considered clinically meaningful for cancer patients with cachexia. In addition to weight gain, the drug also showed promise in increasing appetite and physical activity, which are important measures of wellness for these patients.
Pfizer stated that there were no significant side effects observed with the drug, and treatment-related side effects occurred in a similar percentage of patients in both the placebo and treatment groups.
The company presented the trial data at a cancer research conference and published the results in The New England Journal of Medicine. Pfizer is now discussing late-stage development plans with regulators and aims to initiate further studies in 2025 to support the drug’s approval. They are also conducting a phase two trial of ponsegromab in patients with heart failure, another condition that can lead to cachexia.
Although Pfizer has not disclosed the estimated revenue opportunity for the drug, the positive results from the midstage trial suggest a potential breakthrough in the treatment of cancer cachexia. If approved, ponsegromab could significantly improve the quality of life and survival rates for millions of cancer patients worldwide.