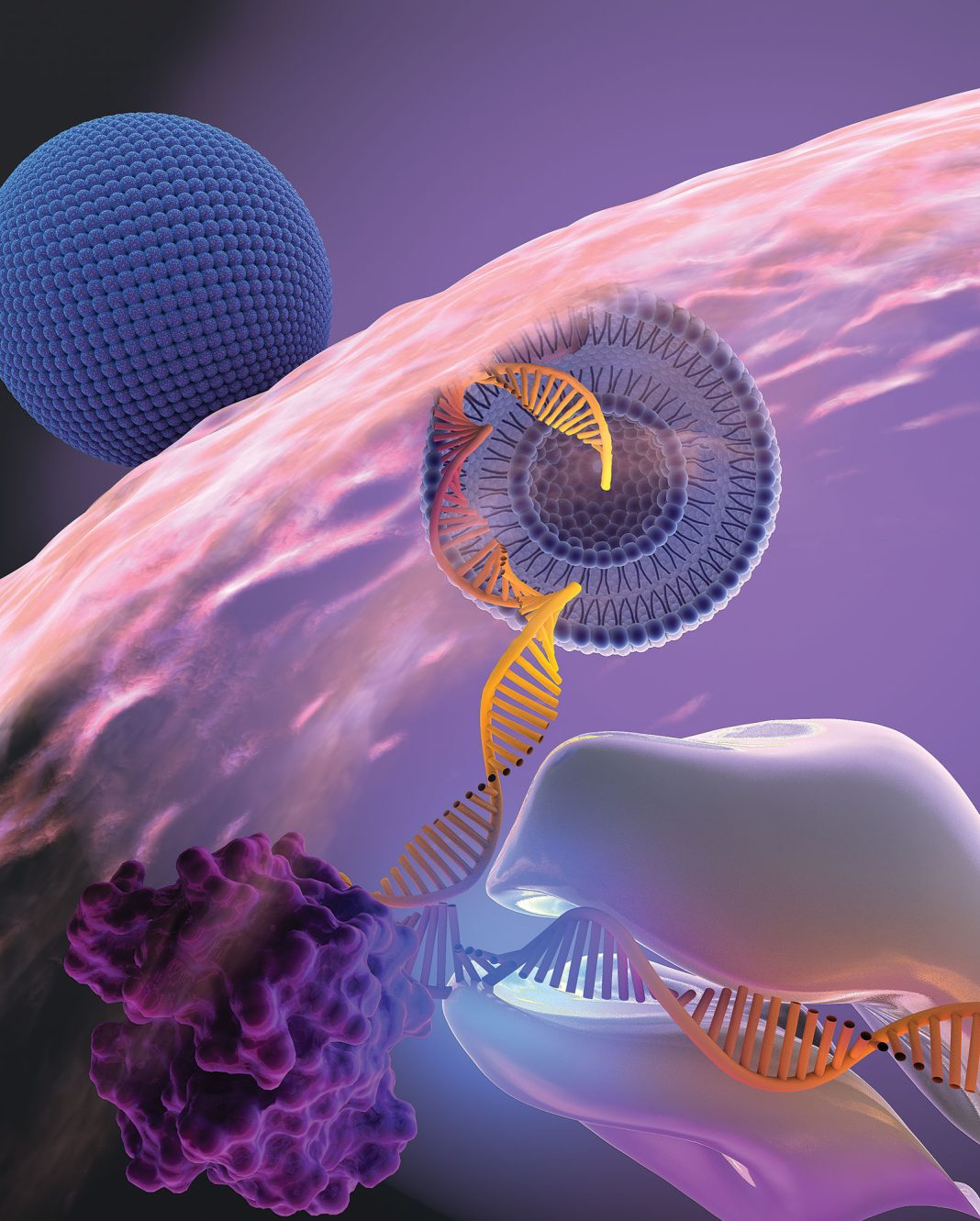
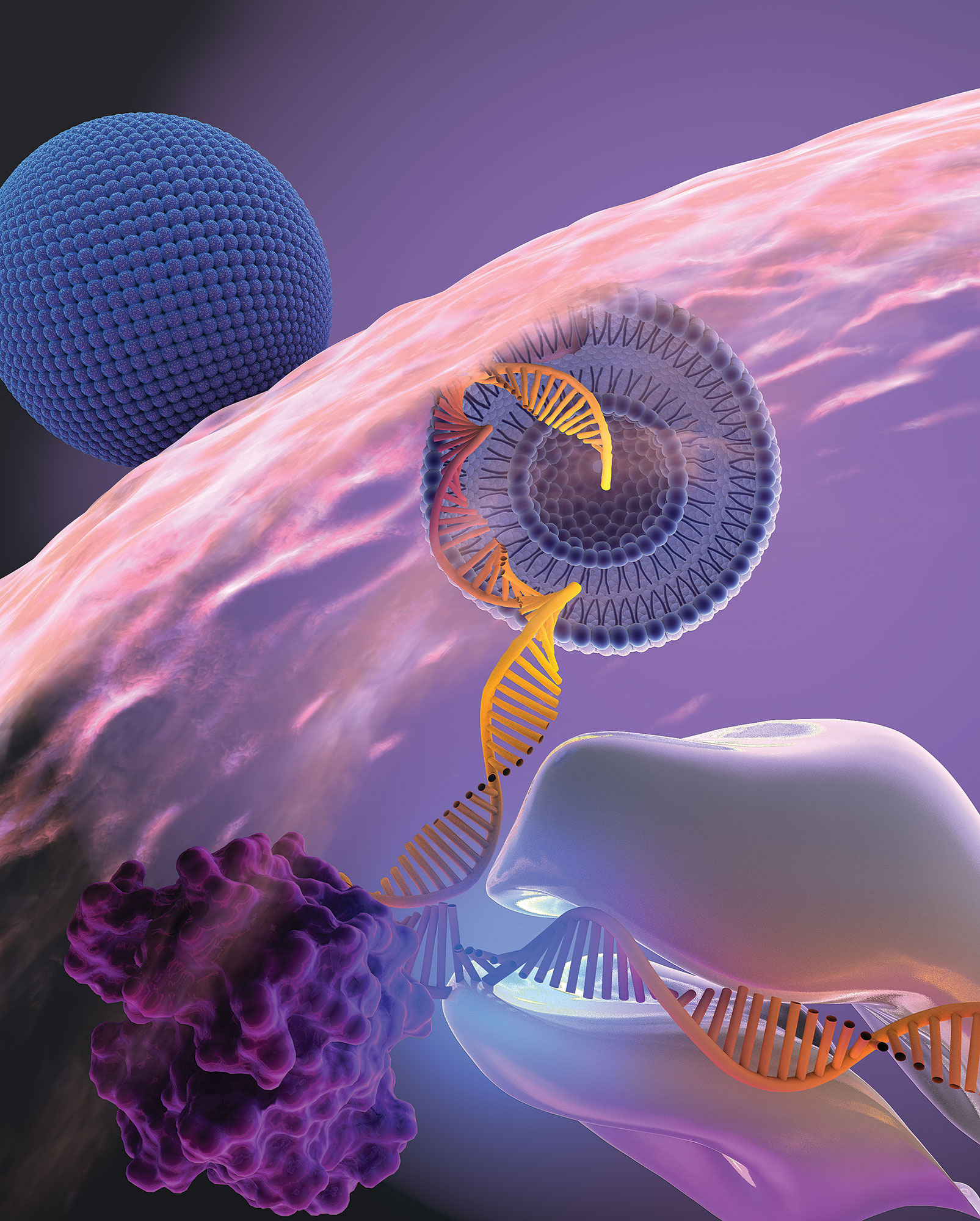
 Moderna, the biotech company at the forefront of COVID-19 vaccine development, has announced promising results from a late-stage trial of its combination vaccine that targets both COVID-19 and the flu. This makes Moderna the first company to release positive phase three data on a COVID-19 and flu combination shot, giving it a potential advantage over competitors like Pfizer and Novavax.
Moderna, the biotech company at the forefront of COVID-19 vaccine development, has announced promising results from a late-stage trial of its combination vaccine that targets both COVID-19 and the flu. This makes Moderna the first company to release positive phase three data on a COVID-19 and flu combination shot, giving it a potential advantage over competitors like Pfizer and Novavax.
Combination shots have been touted as a convenient solution for individuals seeking protection against respiratory viruses that typically surge during the same season. With fewer Americans getting vaccinated against COVID-19, the added convenience of a combination shot could encourage more people to protect themselves against these viruses. Additionally, combination shots have the potential to alleviate the burden on pharmacists and the already stretched-thin US healthcare system.
Moderna’s messenger RNA (mRNA) combination shot, named mRNA-1083, is made up of two experimental vaccines: one for seasonal influenza (mRNA-1010) and a “next-generation” version of its COVID-19 shot (mRNA-1283). Both vaccines have shown positive results in separate phase three trials. The ongoing late-stage trial on mRNA-1083 involved 8,000 patients and compared the combination shot to an enhanced flu vaccine and Moderna’s licensed COVID-19 shot.
The results of the trial are encouraging. In both age groups studied (patients ages 65 and above, and participants between the ages of 50 and 64), a single dose of Moderna’s combination vaccine produced statistically significant higher immune responses against three strains of influenza and the COVID-19 omicron variant XBB.1.5.
In terms of safety and tolerability, the combination shot was deemed acceptable. The most common side effects reported were injection site pain, fatigue, muscle pain, and headache. Luckily, the majority of these effects were mild to moderate in severity.
Moderna is not stopping at the COVID-19 and flu combination vaccine. The company is also developing a combination shot that targets the flu and respiratory syncytial virus (RSV), as well as a vaccine that targets all three respiratory viruses: COVID-19, flu, and RSV.
It is worth noting that other major players in the vaccine development race, namely Pfizer and Novavax, are also exploring combination shots that target both COVID-19 and the flu. However, Moderna’s use of messenger RNA technology sets it apart from Pfizer, which relies on protein-based technology for its COVID-19 shot. Moderna’s mRNA technology has already proven successful in the development of its authorized COVID-19 vaccine, Spikevax.
In conclusion, Moderna’s combination vaccine targeting both COVID-19 and the flu has shown promising results in a late-stage trial. This development could potentially give Moderna an edge over its competitors in the race to provide a convenient solution for individuals seeking protection against multiple respiratory viruses. The company’s use of mRNA technology, which has already demonstrated success in its authorized COVID-19 vaccine, further solidifies its position as a frontrunner in the field of vaccine development.