FDA Seizes Counterfeit Ozempic, Urges Caution
The US Food and Drug Administration (FDA) has issued a warning about counterfeit versions of the type 2 diabetes drug Ozempic that have been seized from the US drug supply chain. The agency is urging suppliers, pharmacies, and patients to exercise caution. Although five people have fallen ill in connection with these counterfeit products, none of the cases have been serious, according to the FDA.
Double-Check Semaglutide Products
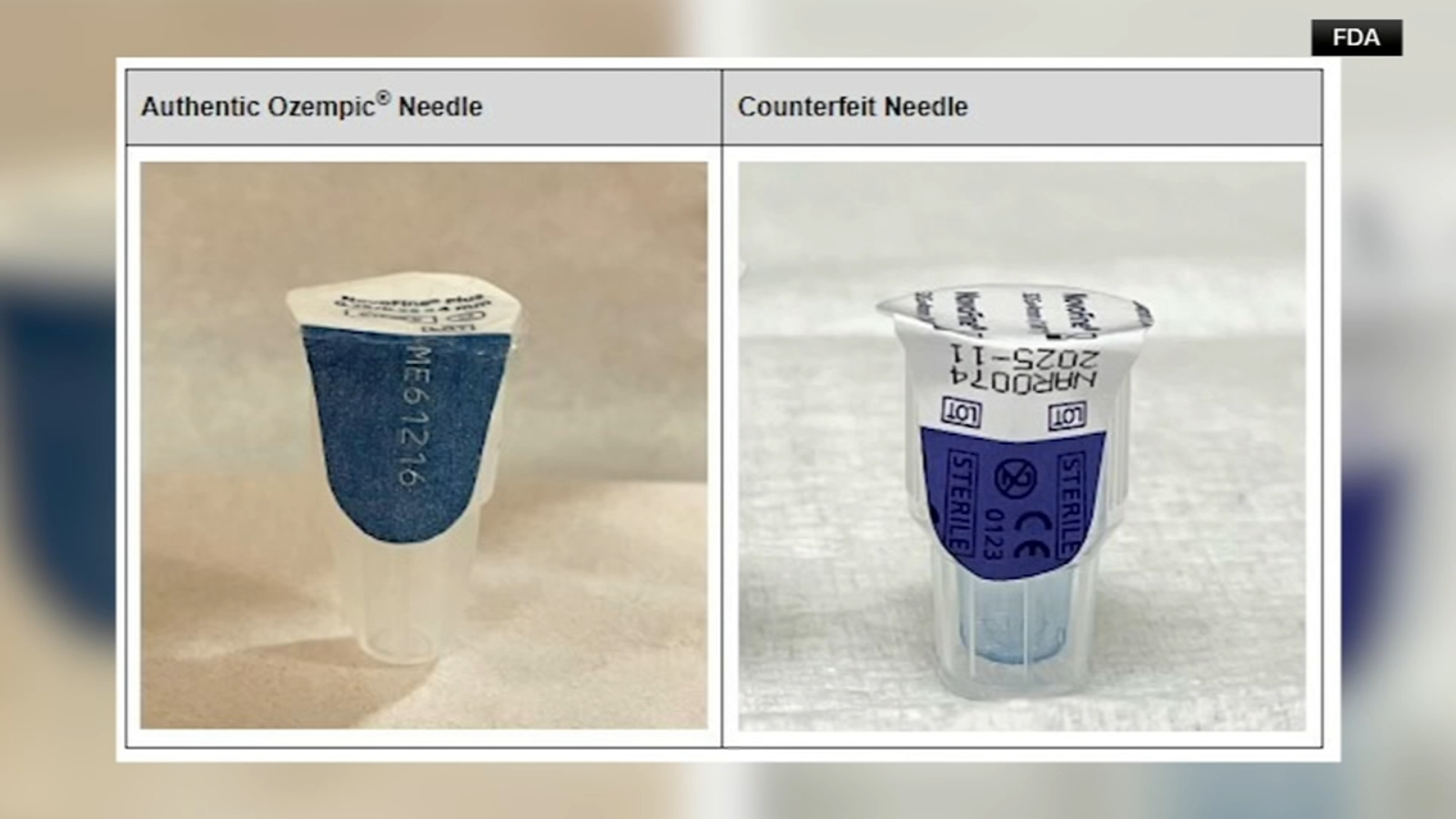
Pharmacies, health care systems, wholesalers, and patients are being advised by the FDA to double-check their semaglutide products to ensure they are genuine. Specifically, one-milligram injectable Ozempic products with the lot number NAR0074 and serial number 430834149057 on the box should not be used.
Fake Products Under Investigation
The FDA, in collaboration with drugmaker Novo Nordisk, is currently testing the counterfeit Ozempic products to determine their potential dangers and identify the substances they contain. Additionally, the pen label, carton, patient information, and needles that come with the injectors are also counterfeit. The FDA warns that the sterility of these needles cannot be confirmed, which may lead to infections if used.
Obtain Medication from Licensed Pharmacies
Patient safety is of utmost importance, and the FDA reminds individuals to obtain their medication only through state-licensed pharmacies with a valid prescription. This ensures the authenticity and quality of the medication.
Counterfeit Ozempic and Wegovy
Ozempic, along with its sister medication for weight loss, Wegovy, has experienced a surge in popularity among celebrities. Consequently, there have been reports of knockoff versions being sold at salons and through social media. To combat this issue, the FDA has been sending warning letters to online sellers, while Novo Nordisk has taken legal action against medical spas, clinics, and weight loss centers selling fake versions.
Collaborative Efforts to Remove Counterfeit Products
The FDA is working closely with other federal agencies and Novo Nordisk to identify and remove additional counterfeit semaglutide injectable products from the market.
Reporting Counterfeit Products
If consumers have questions or come across fake products, they can contact Novo Nordisk at 1-800-727-6500. It is also important to report counterfeit products to the local FDA consumer complaint coordinator or through the FDA’s website.
The-CNN-Wire & 2023 Cable News Network, Inc., a Warner Bros. Discovery Company. All rights reserved.


