 Leqembi, the newly FDA-approved Alzheimer’s treatment, has shown promising results in slowing disease progression in patients over three years, according to new data released by Japanese drugmaker Eisai. The study, conducted in collaboration with Biogen, also found that a patient’s Alzheimer’s disease worsens after they stop treatment. It was observed that rates of adverse side effects associated with Leqembi, such as brain bleeding and swelling, decreased after six months of treatment.
Leqembi, the newly FDA-approved Alzheimer’s treatment, has shown promising results in slowing disease progression in patients over three years, according to new data released by Japanese drugmaker Eisai. The study, conducted in collaboration with Biogen, also found that a patient’s Alzheimer’s disease worsens after they stop treatment. It was observed that rates of adverse side effects associated with Leqembi, such as brain bleeding and swelling, decreased after six months of treatment.
The decline in side effects is significant, as it addresses concerns raised by some doctors and was a major reason why a European drug regulator initially recommended against approving Leqembi. This study provides the longest available efficacy and safety data to date on Leqembi, which has faced challenges in its rollout in the U.S. due to diagnostic test requirements and regular brain scans.
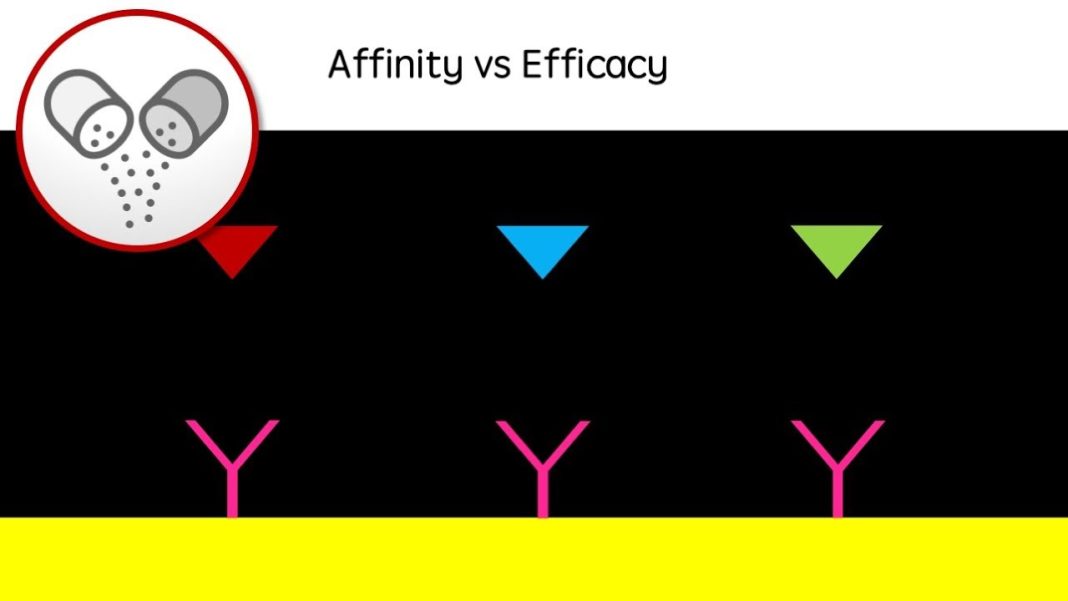
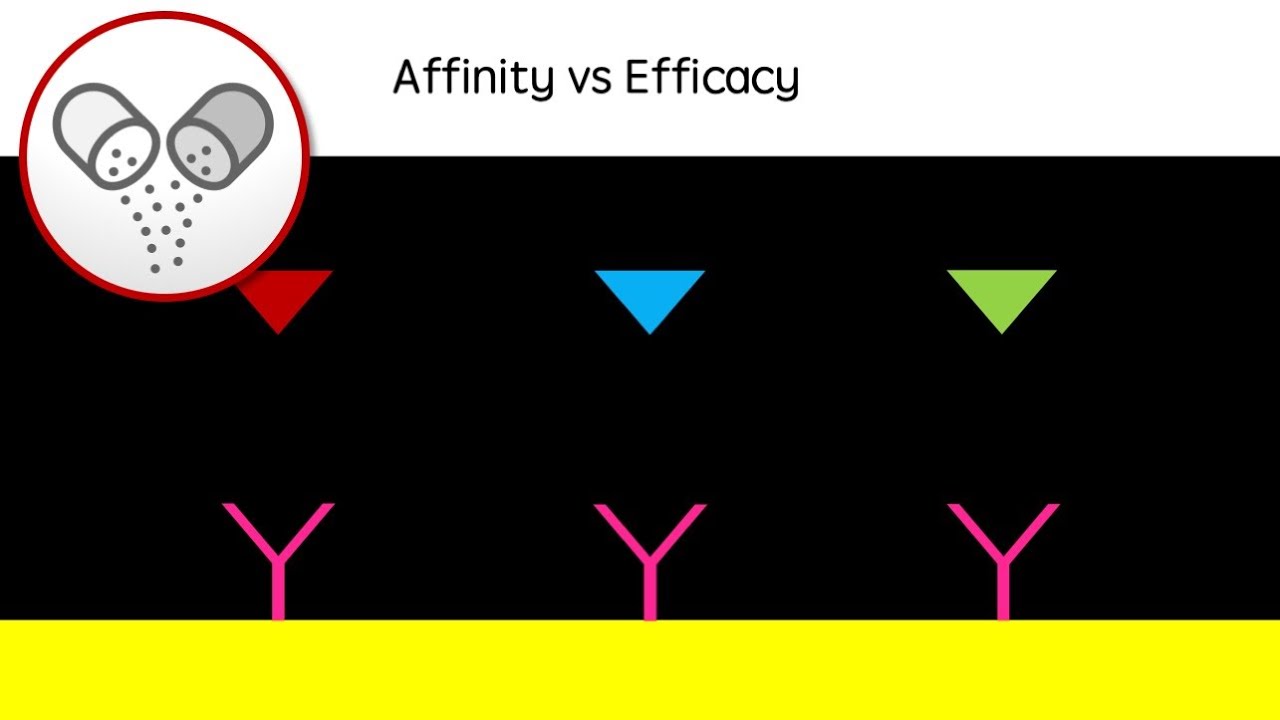
The findings were presented at the Alzheimer’s Association International Conference in Philadelphia, providing a first glimpse into the potential future for Alzheimer’s patients on therapies like Leqembi. The drug, administered through an infusion twice a month, is a monoclonal antibody that targets toxic plaques called amyloid in the brain, which are a hallmark of Alzheimer’s. It works by slowing the progression of the disease during its early stages and clearing the building blocks of amyloid plaque called protofibrils.
Dr. Lynn Kramer, Eisai’s chief clinical officer of deep human biology learning, emphasized the importance of early and sustained treatment for Alzheimer’s patients. While Leqembi is not a cure, starting treatment early can provide years of benefit in terms of maintaining cognition and functionality. Eisai believes that patients may eventually be able to switch to a maintenance dose of Leqembi after 18 to 24 months of treatment, offering a more convenient option for long-term use.
Eisai and Biogen are currently seeking regulatory approval for a once-monthly infusion of Leqembi, with a decision expected in January. They also plan to introduce an injectable form of the drug that patients can administer at home once a week. These developments are expected to make the treatment more accessible and convenient for patients, potentially transforming the paradigm of Alzheimer’s care.
According to the Alzheimer’s Association, nearly 7 million Americans currently have the condition, and this number is projected to rise to almost 13 million by 2050. The results of this long-term study on Leqembi provide hope for better management and treatment options for Alzheimer’s patients.
The study involved participants from mid-stage and late-stage trials on Leqembi. One trial, called Clarity AD, followed three different groups of patients for 36 months. The group that started Leqembi early showed a slower rate of cognitive decline compared to the other two groups. The difference in cognitive decline between the early start group and the group that received no treatment grew larger over time, highlighting the effectiveness of early intervention.
Another sub-study examined patients with low levels of another protein called tau, which is considered a marker of Alzheimer’s severity. After three years on Leqembi, a significant percentage of these patients did not experience any progression of their Alzheimer’s, and some even saw an improvement in their condition.
In a separate trial, patients who temporarily stopped treatment with Leqembi saw their cognitive decline revert to the rate of those who had received a placebo during the gap period. This indicates that even when amyloid plaque is removed, the disease continues to progress if the treatment is discontinued.
Overall, the data from these studies underscores the importance of continuous treatment for Alzheimer’s patients. Leqembi has shown promising results in slowing disease progression, but it is crucial for patients to remain on the treatment to maintain cognitive function and slow the advancement of the disease. The potential approval of a once-monthly infusion and a self-administered injectable form of Leqembi could significantly improve the accessibility and convenience of the treatment, ultimately benefiting millions of Alzheimer’s patients.