 FDA Approves Eli Lilly’s Alzheimer’s Drug
FDA Approves Eli Lilly’s Alzheimer’s Drug
The FDA has granted approval for Eli Lilly’s Alzheimer’s drug donanemab, providing a new treatment option for patients with early symptomatic Alzheimer’s disease. This approval comes as a significant development in the fight against the mind-wasting disease, which affects nearly 7 million Americans and is the fifth-leading cause of death for adults over 65. By 2050, the number of individuals with Alzheimer’s is projected to rise to almost 13 million in the U.S.
The approval of donanemab is seen as a major step forward in the treatment of Alzheimer’s, as it offers patients more options and greater opportunities for extended time. This advancement has been eagerly awaited by those affected by the devastating disease.
Eli Lilly faced obstacles in bringing donanemab to market, with the FDA initially rejecting its approval last year due to insufficient data and then surprisingly delaying it again in March. However, last month an advisory panel recommended the treatment for full approval, highlighting that the benefits outweigh its risks.
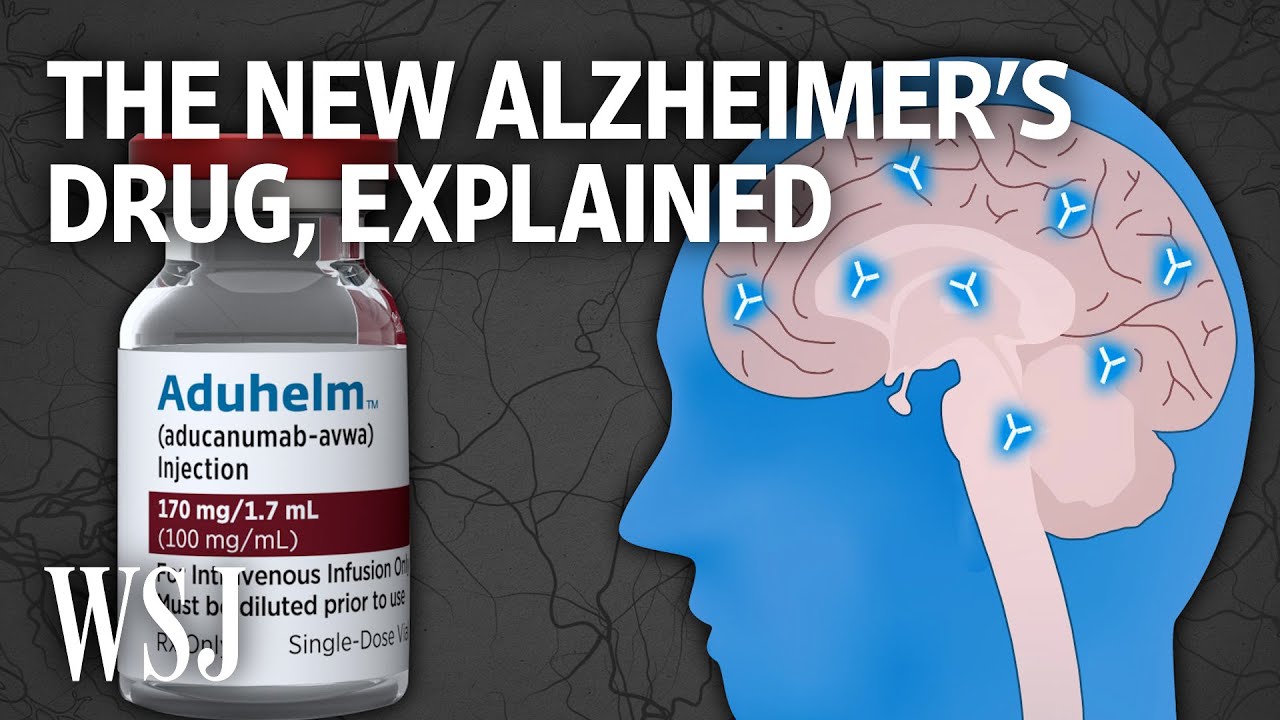
Donanemab will now compete with Biogen and Eisai’s treatment called Leqembi, which was approved last summer. Both drugs are monoclonal antibodies that target amyloid plaques in the brain, a key characteristic of Alzheimer’s. They aim to slow down the progression of the disease in patients at the early stages.
In a late-stage trial, Eli Lilly’s drug demonstrated a 35% reduction in the progression of Alzheimer’s compared to a placebo over 18 months. Patients who achieved certain goals for amyloid plaque clearance were able to transition to a placebo after six, 12, or 18 months of treatment.
It is important to note that neither donanemab nor Leqembi is a cure for Alzheimer’s. Drugs targeting and clearing amyloid plaques carry safety risks, including severe and potentially fatal side effects such as swelling and bleeding in the brain. In fact, three patients in Eli Lilly’s trial died from these side effects.
The cost of donanemab treatment is estimated to be $12,522 for a six-month course, $32,000 for 12 months, and $48,696 for 18 months. Medicare coverage and reimbursement options are available for eligible patients.
Eli Lilly’s donanemab is now the third drug of its kind to reach the market, following the approval of Leqembi and the ill-fated therapy Aduhelm from Biogen and Eisai. The latter was recently dropped by the two companies. The FDA received criticism for its expedited approval of Aduhelm in 2021, despite a negative recommendation from an advisory panel.
In conclusion, the FDA’s approval of Eli Lilly’s Alzheimer’s drug donanemab marks significant progress in the treatment of this devastating disease. While it offers new options for patients, it is essential to consider the potential risks associated with drugs targeting amyloid plaques. Further research and development in the field of Alzheimer’s treatment are crucial to finding more effective and safer solutions for those affected by the disease.