 Opal Sandy, a deaf child, has regained her ability to hear through an experimental gene therapy called DB-OTO developed by Regeneron. Opal received the therapy when she was just 11 months old, and within six months, her hearing was assessed as normal. Her parents have witnessed the incredible transformation as Opal can now hear them and respond with words like “Dada.” This groundbreaking achievement has left Opal’s mother, Jo Sandy, amazed and grateful.
Opal Sandy, a deaf child, has regained her ability to hear through an experimental gene therapy called DB-OTO developed by Regeneron. Opal received the therapy when she was just 11 months old, and within six months, her hearing was assessed as normal. Her parents have witnessed the incredible transformation as Opal can now hear them and respond with words like “Dada.” This groundbreaking achievement has left Opal’s mother, Jo Sandy, amazed and grateful.
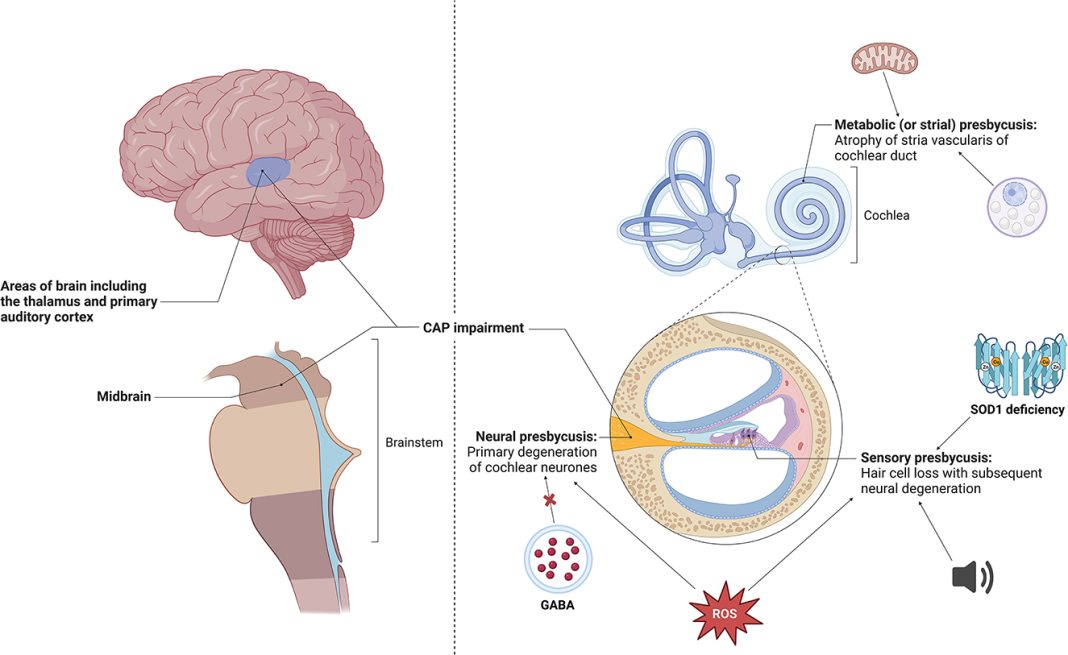
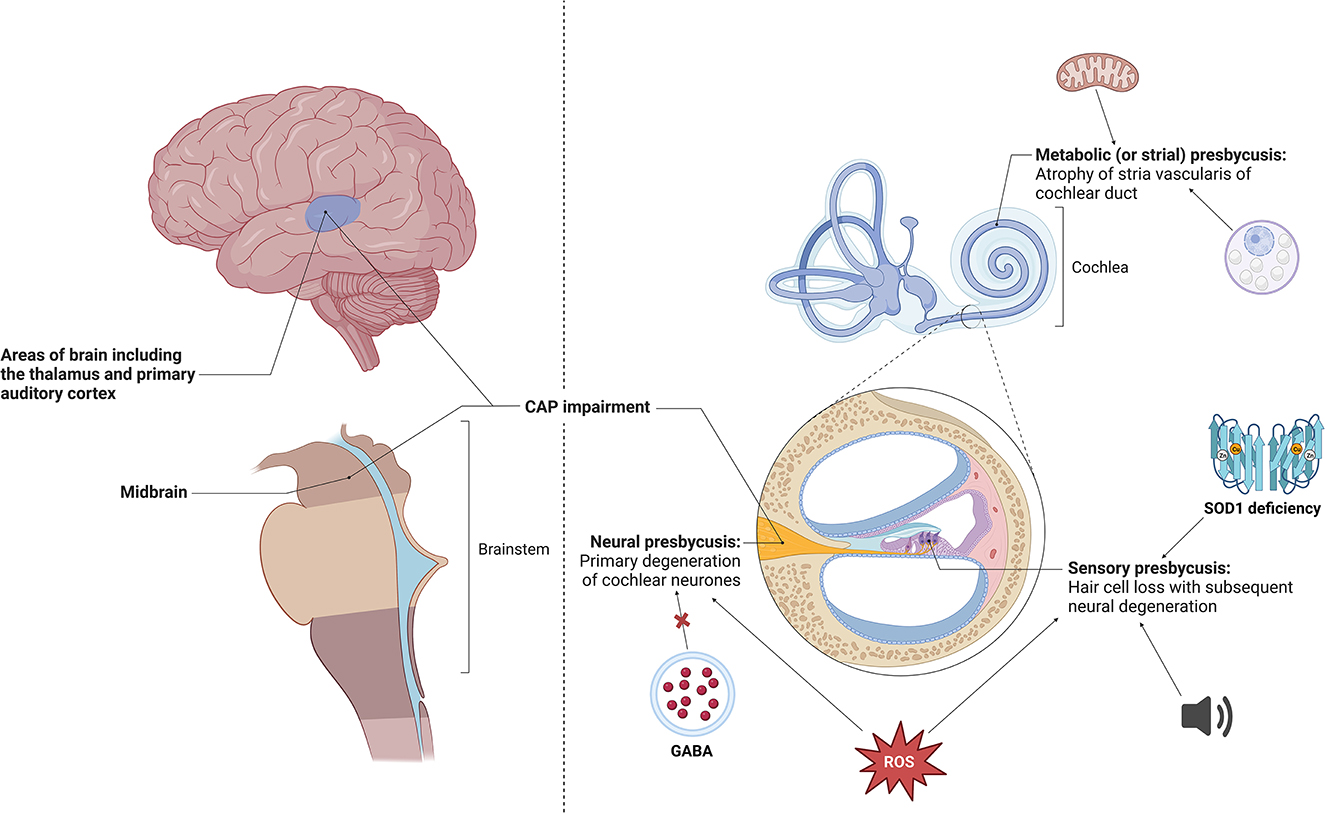
Dr. Lawrence Lustig, one of the trial investigators and the chair of Columbia University’s Department of Otolaryngology, expressed his astonishment at the therapy’s success. He stated that providing the full complexity and spectrum of sound to children born with profound genetic deafness was something he never expected to witness in his lifetime. The therapy’s effectiveness in restoring Opal’s hearing is a significant breakthrough, especially considering that Opal was born with genetic deafness caused by mutations in the otoferlin gene.
Mutations in the otoferlin gene hinder the production of a protein necessary for communication between the inner ear and the auditory nerve. While hearing aids and cochlear implants can partially rectify this problem, the ultimate goal was to restore Opal’s ability to hear all speech sounds. Opal’s father, James Sandy, shared how the therapy has already made a difference in their daily lives, such as during bath-time or swimming when Opal cannot wear her cochlear implant.
The DB-OTO gene therapy aims to restore the full spectrum of sound to individuals with mutated otoferlin genes. It is administered through an intracochlear injection into one ear or directly to the inner ear. A phase 1/2 clinical trial for this therapy began in 2023, enrolling children from the United States, the United Kingdom, and Spain. The trial aims to gather more information about the therapy’s safety and efficacy, measure changes in hearing after the injection, and assess any adverse events experienced by the children.
Regeneron, the company behind DB-OTO, is based in New York state. The therapy utilizes a modified virus that poses no threat to humans and delivers a working copy of the otoferlin gene to replace the faulty one. The success of the therapy has been remarkable, with Opal’s hearing ability being detected just four weeks after the injection and continuing to improve until it was measured as normal around five months later.
Opal’s case is not an isolated incident. Another child who received the injection at the age of 4 also showed improved hearing six weeks after receiving DB-OTO. The results of this therapy have been measured through various tests, including pure tone audiometry, which evaluates the reaction to sounds emitted at different decibel levels. Dr. Lustig emphasized that these impressive results highlight the revolutionary potential of DB-OTO as a treatment for otoferlin-related deafness.
The trial has reported no adverse events related to the therapy among participants, further indicating its safety. As Regeneron continues to seek further testing and enrollment of additional participants, the results of this gene therapy offer hope for individuals with various types of hearing loss. Dr. Manohar Bance, an ear surgeon with the UK’s National Health Service (NHS), expressed his excitement about this breakthrough, stating that it marks the beginning of a new era for gene therapies in otology and audiology.
In conclusion, Regeneron’s gene therapy, DB-OTO, has shown remarkable success in restoring hearing to deaf children with genetic mutations in the otoferlin gene. The therapy’s effectiveness has surpassed expectations, with Opal Sandy and another child showing significant improvements in their hearing abilities. The trial has reported no adverse events related to the therapy, further enhancing its safety profile. This breakthrough brings hope for individuals with various types of hearing loss and signals the beginning of an exciting new era in gene therapies for the inner ear.